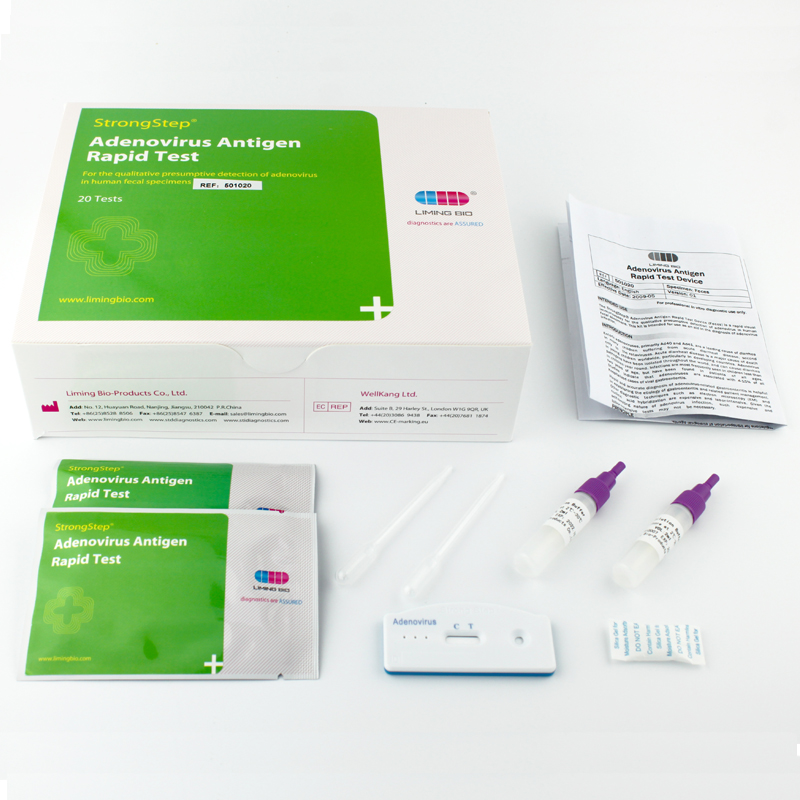
Products Details
INTENDED USE The StrongStep® Adenovirus Rapid Test Device (Feces) is a rapid visual immunoassay for the qualitative presumptive detection of adenovirus in human fecal specimens. This kit is intended for use as an aid in the diagnosis of adenovirus infection. INTRODUCTION Enteric adenoviruses, primarily Ad40 and Ad41, are a leading cause of diarrhea in many children suffering from acute diarrheal disease, second only to the rotaviruses. Acute diarrheal disease is a major cause of death in young children worldwide, particularly in developing countries. Adenovirus pathogens have been isolated throughout the world, and can cause diarrhea in children year round. Infections are most frequently seen in children less than two years of age, but have been found in patients of all ages. Studies indicate that adenoviruses are associated with 4-15% of all hospitalized cases of viral gastroenteritis. Rapid and accurate diagnosis of adenovirus-related gastroenteritis is helpful in establishing the etiology of gastroenteritis and related patient management. Other diagnostic techniques such as electron microscopy (EM) and nucleic acid hybridization are expensive and labor-intensive. Given the self-limiting nature of adenovirus infection, such expensive and labor-intensive tests may not be necessary. PRINCIPLE The Adenovirus Rapid Test Device (Feces) detects adenovirus through visual interpretation of color development on the internal strip. Anti-adenovirus antibodies are immobilized on the test region of the membrane. During testing, the specimen reacts with anti-adenovirus antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient adenovirus in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred. PROCEDURE Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use. 1. Specimen collection and pre-treatment: 1) Use clean, dry containers for specimen collection. Best results will be obtained if the assay is performed within 6 hours after collection. 2) For solid specimens: Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). For liquid specimens: Hold the pipette vertically, aspirate fecal specimens, and then transfer 2 drops (approximately 80 µL) into the specimen collection tube containing the extraction buffer. 3) Replace the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube. 4) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if not tested within 1 hour after preparation. 2. Testing 1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. For best results, the assay should be performed within one hour. 2) Using a piece of tissue paper, break the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the specimen well (S) of the test device.Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the result window. As the test begins to work, color will migrate across the membrane. 3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes. Note: If the specimen does not migrate due to the presence of particles, centrifuge the extracted specimens contained in the extraction buffer vial. Collect 100 µL of supernatant, dispense into the specimen well (S) of a new test device and start again, following the instructions described above. CertificationsAdenovirus 1Adenovirus 2InstructionsMSDS




Qpcr Primer,
PCT testing,
Candida Antigen Combo Rapid Test,
Rapid Std Screening,
Candida Albicans During Pregnancy,
Coronavirus Test Accuracy,
Antigen Test with Dual Biosafety System,
Candida Albicans Is A,
C Trachomatis N Gonorrhoeae,
Rapid Antigen Test For Strep Throat,











