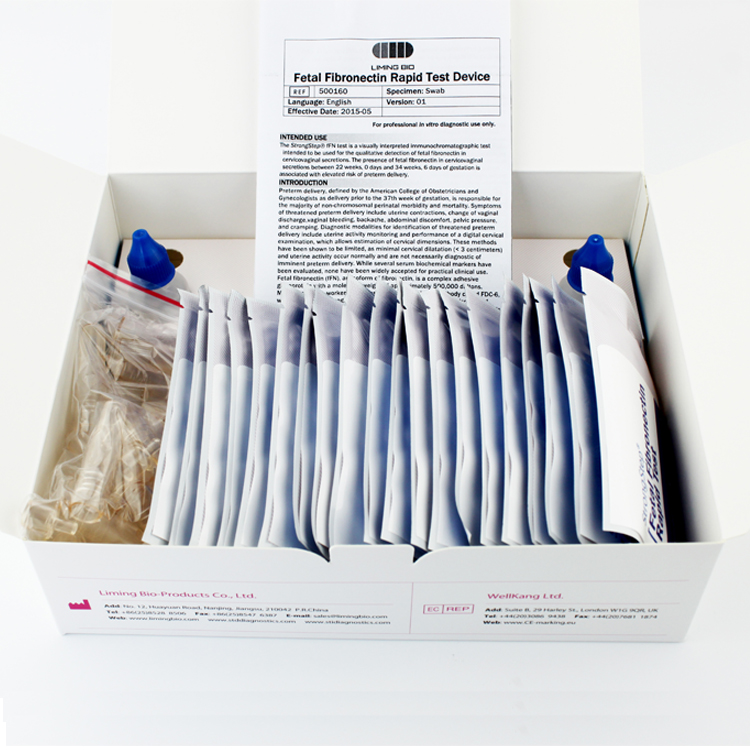
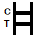| 20 Individually packed test devices | Each device contains a strip with colored conjugates and reactive reagents pre-coated at the corresponding regions. |
| 2 Extraction Buffer vial | 0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| 1 Positive control swab (on request only) | Contain fFN and sodium azide. For External control. |
| 1 Negative control swab (on request only) | Not contain fFN. For external control. |
| 20 Extraction tubes | For specimens preparation use. |
| 1 Workstation | Place for holding buffer vials and tubes. |
| 1 Package insert | For operation instruction. |
| Timer | For timing use. |
| POSITIVE RESULT:
| Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). |
| NEGATIVE RESULT:
| Only one colored band appears in the control region (C). No apparent colored band appears in the test region (T). |
| INVALID RESULT:
| Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. |
| Relative Sensitivity: 97.96% (89.13%-99.95%)* Relative Specificity: 98.73% (95.50%-99.85%)* Overall Agreement: 98.55% (95.82%-99.70%)* *95% Confidence Interval |
| Another brand |
| ||
| + | - | Total | |||
| StrongStep® fFn Test | + | 48 | 2 | 50 | |
| - | 1 | 156 | 157 | ||
|
| 49 | 158 | 207 | ||
| Substance | Concentration | Substance | Concentration |
| Ampicillin | 1.47 mg/mL | Prostaglandin F2 | a 0.033 mg/mL |
| Erythromycin | 0.272 mg/mL | Prostaglandin E2 | 0.033 mg/mL |
| Maternal Urine 3rd Trimester | 5% (vol) | MonistatR (miconazole) | 0.5 mg/mL |
| Oxytocin | 10 IU/mL | Indigo Carmine | 0.232 mg/mL |
| Terbutaline | 3.59 mg/mL | Gentamicin | 0.849 mg/mL |
| Dexamethasone | 2.50 mg/mL | BetadineR Gel | 10 mg/mL |
| MgSO4•7H2O | 1.49 mg/mL | BetadineR Cleanser | 10 mg/mL |
| Ritodrine | 0.33 mg/mL | K-YR Jelly | 62.5 mg/mL |
| DermicidolR 2000 | 25.73 mg/mL |
|
| Catalog number |  | Temperature limitation |
 | Consult instructions for use |
| Batch code |
 | In vitro diagnostic medical device |  | Use by |
 | Manufacturer |  | Contains sufficient for <n> tests |
 | Do not reuse |  | Authorized representative in the European Community |
 | CE marked according to IVD Medical Devices Directive 98/79/EC | ||
 Preterm delivery, defined by the American College of Obstetricians and Gynecologists as delivery prior to the 37th week of gestation, is responsible for the majority of non-chromosomal perinatal morbidity and mortality. Symptoms of threatened preterm delivery include uterine contractions, change of vaginal discharge,vaginal bleeding, backache, abdominal discomfort, pelvic pressure, and cramping. Diagnostic modalities for identification of threatened preterm delivery include uterine activity monitoring and performance of a digital cervical examination, which allows estimation of cervical dimensions. StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions with the following characteristics: User friendly: one-step procedure in qualitative testing Rapid: only 10 minutes required during the same patient visiting Equipment-free: the source-limiting hospitals or clinical setting can perform this test Delivered: room temperature (2℃-30℃)
Preterm delivery, defined by the American College of Obstetricians and Gynecologists as delivery prior to the 37th week of gestation, is responsible for the majority of non-chromosomal perinatal morbidity and mortality. Symptoms of threatened preterm delivery include uterine contractions, change of vaginal discharge,vaginal bleeding, backache, abdominal discomfort, pelvic pressure, and cramping. Diagnostic modalities for identification of threatened preterm delivery include uterine activity monitoring and performance of a digital cervical examination, which allows estimation of cervical dimensions. StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions with the following characteristics: User friendly: one-step procedure in qualitative testing Rapid: only 10 minutes required during the same patient visiting Equipment-free: the source-limiting hospitals or clinical setting can perform this test Delivered: room temperature (2℃-30℃)